How Do They Do That? Wringing Sulfuric Acid out of Sulfur Dioxide Emissions
Copper Applications in Mining & Extraction
One of the bi-products of the smelting process is the gas sulfur dioxide (S0 2). Most industrial facilities make a significant effort to reduce the emission of this gas into the air for environmental reasons. For example, at the BHP Copper Metals smelter at San Manuel, Arizona - the largest copper smelter in North America - a sophisticated capture system captures as much as 99 percent of the sulfur dioxide produced in the process.
What is more, the sulfur dioxide that is retained is put through a rigorous process to make a useful product: sulfuric acid, the largest volume chemical used in the world today.


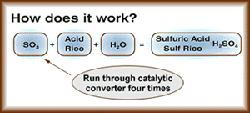 The extremely hot (670 degrees Fahrenheit) and dry dirty gas from the flash furnace and converters, which is 10 percent sulfur dioxide, gets sprayed with water, cooled, cleaned, dried, run through a catalytic converter four times, is converted to sulfur trioxide (SO 3), and finally combined with water (H 20) to make sulfuric acid (H 2SO 4).
The extremely hot (670 degrees Fahrenheit) and dry dirty gas from the flash furnace and converters, which is 10 percent sulfur dioxide, gets sprayed with water, cooled, cleaned, dried, run through a catalytic converter four times, is converted to sulfur trioxide (SO 3), and finally combined with water (H 20) to make sulfuric acid (H 2SO 4).
| As little as 1% of the S0 2is emitted, |
  |
 |
 |
The BHP smelter at San Manuel produces 450,000 gallons of sulfuric acid a day, or enough to fill 12,500 rail cars or 52,000 trucks every year.


 |
 |
 |
 |

| On CU is the quarterly publication of BHP Copper, The Broken Hill Proprietary Corp., Ltd. © 1997 BHP Copper |
|
||||
Also in this Issue:
- How Do They Do That? How Copper is Made
- Traditional Pyrometallurgical Treatment of Concentrate
- How Do They Do That? In Situ Mining
- Millions of Dollars Saved With Non-Traditional Shutdown Procedures
- How Do They Do That? Bringing Copper to Market
- Big Blue Goes Copper
- How Do They Do That? Wringing Sulfuric Acid out of Sulfur Dioxide Emissions