Copper Cleans Up
Copper Applications in Health & Environment
Many industrial operations need water dosed with a chemical cooling agent for efficient operation. In most cases the water/coolant mixture used is recirculated and treated with a variety of chemicals to ensure that algae, moulds and other undesirable organisms do not grow in it. These water/coolant mixtures often contain surface oil and debris from the industrial process. Keeping this recirculating fluid clean is therefore an expensive and ecologically unfriendly operation.
Drinking water, and water for personal use, including water in swimming pools needs treatment to control bacterial growth and prevent transmission of infection from one individual to another. Industrial heating, ventilating and air conditioning units also require recirculating water systems which have to be treated to prevent the formation of scale, corrosion and biological fouling.
Hydrothermal Systems Division, Atlanta, have come up with a technique known as the Bio-Ion® System that involves releasing 1 part per million of copper (1ppm Cu) and 3 parts per billion of silver (3 ppb Ag) into the recirculating water which replaces the chemical treatments currently used. A little copper goes a long way in combating the growth of unwanted organisms in the water.
Industrially, recirculating waters are treated with a variety of strong chemicals to remove and control the growth of algae, bacteria and fungi. Chlorine is commonly used in swimming baths and has quite a pungent smell, and in many cases causes the eyes to sting. For large scale operations chlorine cylinders are used. The chlorine reacts with water to form hypochlorous acid and chlorine ions.
Cl 2(gas) + H 2O (water) » HOCl + H + + Cl –
For small scale operations chlorine cylinders are inconvenient and dangerous to handle. In such cases the hypochlorous acid (HOCl) is generated from calcium hypochlorite, Ca(OCl)2 by reaction with water. Problems arise when the acidity of the water is not closely monitored. Too high an acidity can cause corrosion to circulating equipment, whilst too low an acidity promotes the formation of ammonium derivatives, especially NCl3 that is a powerful eye irritant.
In drinking water, chlorination is used, except when there is present a high concentration of phenol, or one of its derivatives. Chlorine will then react with the phenols to produce chlorinated phenols that have an offensive odor and are toxic. If phenol content is high, chlorine is not used and chlorine dioxide gas is substituted. Chlorination will also result in the production of a group of organic compounds known as trihalomethanes, the most common of which is CHCl3, chloroform. Chloroform can be produced by the reaction of hypochlorous acid with the organic pollutants in the water. Chloroform levels have to be kept below 100 ppb for safety in drinking water.
More recently biocides have been introduced into water purification. These work well but pose threats to the personnel involved in using them. When water has subsequently to be disposed of, the biocides present an ecological danger, and have to be dealt with professionally to avoid contamination of rivers and watercourses.
On the other hand you and I need copper to stay healthy, as it is needed in the formation of enzymes in the body which control how the body gives us energy to work, and in the function of the nervous system. There are problems associated with copper deficiency however that can be remedied by taking copper-containing tablets as a nutritional supplement.
The innovation described here consists of a method of releasing copper into the recirculating water system in a controlled manner. Figure 1 shows diagrammatically what the device looks like.
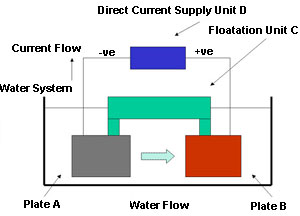 Figure 1. Releasing copper into the recirculating water system
Figure 1. Releasing copper into the recirculating water systemThere are two plates A and B suspended in the water system below a floatation device C. This arrangement keeps the two plates away from any surface debris and oil that might interfere with their operation. The floatation device C is mechanically connected to the sides of the water system to prevent movement by the water flow. In a recirculating system plate A would be upstream of plate B. Plate A is connected to the negative terminal of a direct current supply D, and plate B is connected to the positive terminal of the same direct current supply D. The second plate B is the more important one and is made of copper and silver. The two plates, A and B, are electrodes and the water is the electrolyte in an electrolytic bath, acting in principle, exactly like an electroplating bath.
In operation, when the direct current device is switched on, a small current flows through the circuit. This causes copper and silver to enter into the water at the surface of plate B, as ions through the reaction
Cu 0 » Cu 2+ + 2 e –1
Ag 0 » Ag 1+ + 1 e –1
What this means is that a copper atoms gives up 2 electrons to produce a copper ion and silver atoms give up one electron to produce a silver ion. If the water, that is the electrolyte, were still, these ions would travel across to the other electrode, plate A, and would cover that plate with copper as in a conventional copper-plating bath. The difference is that in this case the flow of the water carries the copper ions away from plate B and ensures that they travel completely round the system. In this way the electrode, plate B releases copper ions and silver ions into the water at a rate that is controllable by direct current source D through a suitable electronic circuit.
Plate B consists of copper and silver such that the ratios of Cu to Ag are between 1 and about 4, that is the Cu/Ag ratio is 1/1 to 4/1. The plates are arranged parallel to the flow of the water to ensure that the Cu ions are swept easily from the plate surface and into the main body of the system.
Cu and Ag are released into the water to produce concentrations of up to 5 ppm Cu and up to 40 ppb Ag, well below the EPA drinking water standard.
So how do we know that the bacteria in the water are being killed off and controlled? In a laboratory it would be possible to use a rapid bacteria count analyzer and determine the extent of the bacteria in about 15 minutes. Unfortunately this is not possible in an industrial environment where such pieces of equipment normally do not exist. A simple test has had to be devised to overcome this problem. What is used are 'Bio-Strips'. These are strips of plastic coated with a medium that will feed any bacteria and make them grow. The strip is placed into its transparent plastic holder and left for up to 48 hours at room temperature. Any bacteria present will grow and develop a red spot on the strip. By comparing the spots on the strip with a standard color strip the bacteria count can be determined. This sort of test is adequate, for instance, for the day-to-day control of a circulating system on lathe used to machine metal parts. The standard color strip provided has been developed by Hydrotherm Systems by carrying out controlled bacteria growth experiments and measuring the bacteria count accurately with a rapid bacteria count analyzer.
When first used, the power unit is turned full on to release copper and silver ions into the water. The bacteria count is taken until it reaches the desired level for the application. At this stage the powder output is reduced in steps until the biological count is increasing slightly. The output is then increased slightly to hold the desired bacteria count.
It is well known that copper is toxic towards bacteria. A recent article in Innovations reprinted from Diagnostic Medicine compared the effectiveness of brass and copper hardware (door knobs, push plates) with that of stainless steel. The conclusion was that although stainless steel appears clean it would support bacterial growth, whereas copper disinfected itself in about 15 minutes and brass in about 7 hours. In a different field copper-nickel clad plates were used by the International Copper Association (ICA), to clad Florida fishing boat hulls. The cladding prevented the growth of crustaceans on the hull reducing maintenance and also drag through the water. The first effect saved time and money and the second increased fuel efficiency.
Copper vessels have been used for the storage of water in part because copper is formed easily into hollow vessel, and in part because the copper keeps water free from growth so that it remains healthy to drink.
Bordeaux mixture, of which copper sulphate is the main ingredient, has been used as a fungicide since the early 1880s, especially to treat grape vines and orchard trees.
The current innovation is an extension of this effect, using copper in solution to kill bacteria in the water.
As evidence of the efficiency of copper and silver at killing bacteria a sample was taken from cooling tower water that was heavily contaminated with bacteria and added to a 5 gallon tank. Nutrients were added and the sample held at 80ºF for 24 hours. After this time the bacteria had grown such that there were in excess of 10 7 per cc of liquid. The Bio-Ion system was then switched on and the results are shown in the following table.
| Time in hours* | Bacterial Population per cc |
|---|---|
| 0 | 1 x 10 7 |
| 24 | 2 x 10 6 |
| 28 | 1 x 10 6 |
| 72 | 1 x 10 3 |
| 96 | 1 x 10 2 |
| * Number of hours after Bio-Ion System was switched on | |
Considering that a bacterial population of 10 3 or less per cc of solution is looked upon as excellent control for industrial systems this is an impressive performance. Field studies with cooling water tower systems and water/coolant mixtures have confirmed this result. Bacterial populations continue to decrease with continued use, often approaching undetectable levels.
So, what are the other advantages of the new system?
The level of the copper and silver ions are well below the EPA drinking water standards when used with potable water systems. In recirculating water/coolant systems odors are eliminated, and the coolant used is degraded to a lesser extent. Copper and silver ions are more effective than biocides which themselves can be corrosive towards the recirculating system. There is no need for stringent regulations to control the use of copper and silver ions in solutions. The ions are some 1000 times more effective at killing bacteria than chlorine. There are no disposal problems associated with the Bio-Ion technique as there are with other coolant additives, especially biocides. Control is simple and does not depend on the purchase of sophisticated analytical equipment. Savings can be made due to the lack of downtime due to bacteria causing coolant failure. The systems are cheap to run.
There are situations in which the upstream electrode, plate A can be fouled possibly by other metal ions in solution plating out onto it. In this case, it is possible for the direct current unit to be electronically switched at set time intervals, (typically 10 seconds, depending on the application), so that the positive electrode becomes the negative electrode and vice-versa. Both electrodes are then made of the copper-silver material and both release copper and silver ions into solution to prevent fouling of themselves and the water system.
 Figure 2. Direct current supply and control units.
Figure 2. Direct current supply and control units.Hydrothermal Systems are now producing units capable of treating systems from 100 to 50,000 gallons. Electrodes are generally small, 3½ x 1 x ¼ to 3½ x 15 x ¼ (inches) and arranged in sets of 2 to 6. Illustrated in Figure 2 are the direct current supply and control units to treat systems from 200 and 4000 gallons, showing how simple they are. There are several arrangements of the electrodes; up to 200 gallons the immersion electrodes are in the tank itself. Over 200 gallons, the electrodes are in flow-through cells which supply treated water to the main tank from water diverted into the flow-through cells.
The new system, depending on small traces of copper in solution, is environmentally friendly, removes the use of difficult-to-handle chemicals, kills bacteria rapidly, stops odors, is simple to control and maintenance free, and reduces running costs of coolant treatment. The levels of copper are also well within the EPA drinking level standards. In all, an imaginative use of the biocidal effects of copper.
Innovations wishes to thank Mr. George S. Glenn, of Hydrotherm Systems Division, GSG Associates Inc., for the information and illustrations used in this article.
Also in this Issue:
- Brief Early History of Brass
- Introduction to Brasses (Part I)
- Speeding-up Your Computer in the 21st Century Using Copper ICs
- Spotlight on Copper In Architecture
- Introduction to Brasses (Part II)
- Copper Cleans Up
