Copper Hull Sheathing Foils Barnacles
Copper Applications in Industrial & Marine Applications
Background| Copper & Copper Alloy Particulate Systems | Copper Alloy Foil Systems | Application| Problem Solved?| Acknowledgments
Geoff Greetham shows in this article that copper-nickel alloy C70600, when applied to a hull surface, will provide excellent corrosion and biofouling resistance in seawater. When the copper-nickel alloy is provided in the form of a suspension in an epoxy resin, the result is good, and with modern adhesive technology, the Cuproguard system seems to have finally provided a thoroughly cost-effective and labor-saving DIY solution for antifouling treatment.
Background 
Copper and some of the copper alloys, in particular the copper-nickel alloys, have been demonstrated to be highly corrosion resistant in marine environments. Their seawater corrosion resistance and antifouling behavior has been thoroughly documented by C. A. Powell and H. T. Michels, and C. A. Powell. These authors have also reviewed the application of these alloys as cladding or sheathing of ships hulls (and also offshore structures). All four articles contain comprehensive lists of references.
The British Admiralty first used sheets of copper nailed to the bottom of wooden ships as antifoulants with the frigate, Alarm, in 1761. It was some 20 years later that copper nails were used instead of iron nails as the latter caused galvanic corrosion of the iron, resulting in the loss of the copper plates. The need to avoid galvanic action between copper or copper-nickel antifouling sheathing and other exposed metallic components still remains.

Figure 1. The Copper Mariner.
It was not until 1941 that a copper-nickel alloy was used as an antifoulant on a 44-ft (13.4-m) yacht, Miss Revere, on which sheets 0.08 in (2 mm) thick were applied. This was followed in 1968 by the construction of the Asperida. Over the following decade, the International Copper Research Association (INCRA - now the International Copper Association), the International Nickel Company and the Copper Development Association Inc. undertook a development program that culminated, in 1971, in the construction of what has probably become the best-known copper-nickel clad vessel, the 67-ft (20.4-m) shrimp trawler Copper Mariner, ( Figure 1). In this case, 1/4-in (6-mm) thick sheets of 90-10 copper-nickel, UNS Alloy C70600, were welded onto a steel frame.
Data collected from the use of copper-nickel alloy hulls on the Copper Mariner and the many vessels that followed established that the alloys are highly effective in preventing biofouling of the hull. (The vessel remained essentially free from fouling for the first 11 years of service, despite the vessel's use in fouling-prone tropical waters.) The copper-nickel hull resulted in lower maintenance costs, lower fuel costs (due to lack of drag), and a net cost saving over the life of the vessel, even when the high cost of fabricating the copper-nickel hull was taken into account. Fabrication, in this case and others cited over the period in question (1941 - 1991), invariably involved the welding of plates to form a hull, with plate thickness varying from 0.08 in to 0.37 in (2 mm to 9.5 mm).
Although copper-nickel alloys were proven to have both excellent corrosion resistance and superior antifouling properties, the welding technology used at the time was, in a practical sense, only applicable to large vessels, or, possibly, in the initial construction of smaller yachts. There remained the problem of providing the benefits of the copper alloys to the many hundreds of thousands of smaller, mostly nonmetallic boats, yachts, and motor cruisers already in service. How could these vessels be effectively protected and freed from the need (and cost) of periodic dry docking, cleaning and recoating with an expensive antifouling paint? The paint, in fact, gave rise to a second problem: Antifouling marine paints have historically been based on copper-containing pigments as their active ingredient. In recent years, purportedly improved paints based on tributyl tin (TBT) made a strong incursion into the market. Due to its release of toxins to the marine environment, that compound will be banned internationally in 2003, and TBT paints will no longer be available.
What was needed was some type of retro-treatment involving the use of copper-nickel. Ideally, for those so inclined, the average, competent boat owner should be able to apply the treatment effectively. Necessity being the mother of invention, it was at this time that two distinct types of treatment began to be developed. Both use copper or copper-nickel, in sheet or particulate form, respectively.
Copper & Copper Alloy Particulate Systems 
The 1980s saw the development of Copperbot, which consists of metallic copper particles suspended in an epoxy resin. To apply, the suspension is simply brushed, rolled or sprayed onto a thoroughly cleaned hull. After drying, the top surface was abraded to reveal metallic copper at the surface, thus providing the desired fouling resistance. The epoxy resin initially used was water-soluble and was subject to osmosis, a condition in which water penetrates the coating and enters the surface layers of the fiberglass hull causing degradation of the hull structure.
A reformulated and renamed product called Coppercoat, in which the epoxy resin was waterproof, was introduced in 1991. An alternative product known as the Copperbot2000 system uses the original water-miscible epoxy resin over an initial coating of a nonpermeable resin, thus preventing osmosis. VC 17M, a suspension of copper powder in a Teflon film was subsequently developed for DIY use using a roller or spray.
Copperguard 80 was introduced in 1995. It also contains copper particles in an epoxy resin; a related product called Copperguard 90 contains particles of a copper-nickel alloy.
The metallic particulate content of the resins in all of the liquid coatings is a compromise between providing as many metallic particles as possible exposed at the surface after final abrasion and the need to be able to apply the suspension with a roller or spray. This results in only about 30% of the hull surface showing metallic particles to the seawater. The coatings are typically 200 microns in thickness. These particulate-containing resin coatings are effective but have to be applied with care, ensuring that the surface preparation is adequate and that the suspension does not settle during application. Hulls need recoating after about 5 to 10 years.
One alternative to copper or copper-nickel powder suspensions is the use of thin sheet fixed to the outside of the hull. Corrosion testing of 90-10 copper-nickel alloy (UNS C70600) has shown that, depending on seawater conditions, (temperature, flow rate, etc.), corrosion rates can vary from 157 µin to 472 µin (4 µm to12 µm) per year in the first year of exposure, falling to between 78 µin to 113 µin (2 µm and 3 µm) per year after about 7 years. Consequently, even the 0.08-in (2-mm) thick plates used back in 1941 were significantly thicker than necessary for adequate life, and modern hull sheathing does, indeed, utilize much thinner copper or copper-nickel sheet.
Copper Alloy Foil Systems 
By early 1980 the inventor, Frederick Mitchell, had proposed using 0.006-in (150-µm) thick 90-10 copper-nickel alloy (UNS C70600) sticking it to the outside of the hull. It met with mixed success until Mitchell had perfected the foil/adhesive system to his satisfaction. The system was mainly known as Mariner 706. Two ferries in particular were studied in New Zealand over the period 1993 to 1999 and were reported in detail by L. H. Boulton, C. A. Powell and W. B. Hudson. The foil was applied in panels approximately 210 mm by 500 mm (8.27 in X 19.7 in) with a 0.59-in (15-mm) overlap. The general conclusions were that the adhesive-backed foil was effective in preventing biofouling provided that care was taken to prevent galvanic coupling with other metallic fittings. Overall, the use of Mariner 706 on all hulls fitted with the developed product has apparently shown good performance provided that the foil was within specification and correctly applied.
An alternative but significantly different approach was taken by J. Kelly, who applied for a Great Britain patent in August 2001, following it with an International Patent Application in July 2002. Both patents utilize 0.006-in (150-µm) thick copper-nickel sheet. His innovation was to divide the copper-nickel sheet into discrete 0.2-in (5-mm) squares. That difference may not sound significant, but it offers two distinct advantages. The first is that galvanic action is minimized because each square is electrically separated from the next; and if corrosion does occur, it would result in the loss of a few squares only. The second is that sheets of small squares are also more pliable and easier to mold to the shape of a hull and hull fittings.
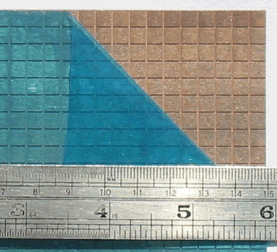 Figure 2. Cuproguard with top protective sheet peeled back.
Figure 2. Cuproguard with top protective sheet peeled back.There are several variants of the system to cater for marine and industrial applications. Cuproguard is manufactured and marketed by Ecosea Ltd., Chandlers Ford, Hants, U.K. To make the finished product, the copper-nickel sheet is brought in as 11.8-in (300-mm) wide foil and stuck onto a two-sided adhesive tape. The tape itself is a sandwich structure consisting of a lower protective film over a pressure-sensitive adhesive layer, a central carrying film, and another pressure-sensitive adhesive layer under an upper protective film. The copper-nickel sheet is applied by simply removing one of the protective films and adhering the metallic film to it. To form the grid, the top surface of the copper-nickel sheet is dry-film resist coated, a photographic image of the grid imposed, and the sheet subsequently etched. The result is an array of discrete copper-nickel squares on a pressure-sensitive backing, the rear (sticky) face of which is protected by a bottom protective film. The top surface is then covered with a thin plastic sheet for handling purposes. Figure 2 shows a panel with the top protective film pulled aside.
Considerable development work was carried out by HiBond Chemicals to determine the optimum formulation for the marine application. Extensive peel, tensile and shear testing was undertaken to confirm the choice.
Application 
After properly preparing the hull surface, the bottom protective film of the sheathing panel is removed to reveal the pressure-sensitive adhesive, which has a low degree of tackiness to aid positioning on the hull. After positioning, a roller is used to activate the adhesive and effectively bond the panel onto the hull. Each panel is butted to the next panel with no overlap. After all panels are applied, the top protective film is removed and the spaces between the copper-nickel squares filled with epoxy resin. Surplus epoxy is wiped away, and washed down whilst still touch-tacky with a nylon pad and warm soapy water to remove any epoxy surface film masking the copper-nickel tiles. The result is a smooth hull surface covered with copper-nickel squares separated by a water-resistant grouting.
The system has an added advantage in that the central plastic film in the adhesive tape is impervious to water. The epoxy grouting is also water resistant; therefore it is very unlikely that water will penetrate the coating system, and the osmosis problem that plagued glass fiber-reinforced hulls earlier is completely avoided. In addition to glass fiber-reinforced plastic hulls, the system can be applied to hulls (and structures) made from steel, concrete, ferro-concrete and wood, again, providing the correct surface preparation has been performed.
 Figure 3. Slim Chance coated with Cuproguard.
Figure 3. Slim Chance coated with Cuproguard.Initial trials of the new foil product were carried out in 1998/1990 by coating the rudder of the yacht Slim Chance. One side of the rudder was coated with copper, the other with copper-nickel. As with the Copper Mariner, biofouling was not observed on either side after at least 11 years (the most recent examination), but the copper-covered side developed a slightly roughened surface, possibly due to erosion-corrosion; whereas, the copper-nickel side remained smooth. Following the granting of a Smart Award from the U.K. Department of Trade and Industry (DTI), the entire hull of Slim Chance was protected with Cuproguard in Spring 2001. The application was described in detail in the popular magazine, Sailing Today, April 2002, pp 73-75. Figure 3 shows the yacht fully coated.
Laboratory testing was also carried out by The Centre for Environment, Fisheries and Aquaculture Science (Burnham on Sea), Essex, England, to confirm the environmental friendliness of Cuproguard. Additionally, sea trials at five fixed sites are in progress and are confirming the excellent corrosion resistance and biofouling resistance of alloy C70600.
What about costs, which are an important issue and the driving force (or impediment, as the case may be) behind a solution to the biofouling problem. Cost estimating is always difficult, but some idea can be gained by making certain assumptions. These are:
- No labor costs included, since the application is all DIY
- For a 36-ft (11-m) yacht, the coating is amortized over a life of 20 years
- For comparison, an antifouling paint system requires that the hull be dry docked, pressure washed, its surface prepared and repainted every year
- Again for comparison, a copper particle system is applied in years 1, 5, 10 and 20, and the hull dry docked, pressure washed and surface-abraded in intervening years
- Cuproguard is applied in year 1 only
- Cuproguard hull dry docked in years 5, 10, 15 and 20 and pressure-washed only
- Prices, determined for the U.K. and converted to U.S. dollars at the exchange rate prevailing in mid-2002, do not include VAT
Roughly speaking, Cuproguard costs total one-half that of the copper particle system and one-third that of the antifouling paint system, taken over 20 years. Probably of equal importance to cost to the average boat owner is avoidance of the yearly need to spend time and energy cleaning, preparing for repainting or surface abrading the hull throughout the 20-year period.
Problem Solved? 
There seems little doubt that copper-nickel alloy C70600 -- when applied to a hull surface by whatever means, and provided that there are no galvanic coupling effects -- will provide excellent corrosion and biofouling resistance in seawater. When the copper-nickel alloy is provided in the form of a suspension in an epoxy resin, the result is good, but it is not as long lasting as when the whole surface of the hull is covered by the alloy. Hence, adhesive attachment seems to be the answer, and with modern adhesive technology the Cuproguard system seems to have finally provided a thoroughly cost-effective and labor-saving DIY solution for antifouling treatment. Time will tell whether the adhesive fails; the copper-nickel alloy certainly will not!
Acknowledgments 
Thanks are due to Chris Stockton, of Ecosea Ltd, for information on Cuproguard, for the sample of Cuproguard and the illustration of the Slim Chance hull.
The following are Trade Names:
Cuproguard - Ecosea Ltd;
Copperbot - Copperbot 98 Ltd;
Copperbot 2000 - Wessex Resins and Adhesives Ltd;
Copperguard 80 and Copperguard 90 - Synthetic Solutions Ltd;
Coppercoat - Aquarius Marine Coatings Ltd;
VC 17M - International Coatings Ltd.
Also in this Issue:
- Copper Hull Sheathing Foils Barnacles
