Will Copper Sop Up Radioactive Pollution
Copper Applications in Health & Environment
Innovations published a series of articles on the subject a few years ago, like "Copper's Role in the Safe Disposal of Radioactive Waste", or "The Swedish Program for Long Term Isolation of High Level Nuclear Waste in Copper Canisters".
But spent fuel disposal is only one of the technologies in which copper can play a meaningful role. An article in the October 15, 2001, issue of Chemical & Engineering News (C&EN) reports that copper may also help get rid of an equally hazardous form of radwaste: cesium-137. 1
A significant quantity of that isotope was formed as a byproduct during decades of plutonium production at the Department of Energy's (DOE) Hanford Site in Richland, Washington, where it is now found in the more than 50 million gallons of liquid waste stored in 177 underground tanks at the site. Some of those tanks are leaking, allowing the cesium to enter the environment. Cesium-137, or 137Cs, is particularly nasty because living organisms easily absorb it, mistaking it for harmless potassium. Also, the isotope has a 30.2-year half-life, meaning that hazardous quantities will remain in the environment for centuries. On the other hand, 137Cs is also an emitter of intense X-rays, possibly giving it life-saving uses in radiotherapy.
Retrieving Hanford's cesium and making it available in a clinical form would therefore benefit both the environment and medical science. 2
A new copper-based material might make such retrieval possible, thanks to research conducted at the Pacific Northwest National Laboratories (PNNL), another DOE facility in Richland. PNNL researchers have created a highly selective sorbent (C&EN calls it a "super sponge") that rapidly removes almost all cesium from aqueous solution.
The sorbent is an organic-inorganic nanocomposite, one of a class of materials known as self-assembled monolayers on mesoporous supports, or SAMMS. Mobil Corporation chemists first produced the materials about 10 years ago, and PNNL and Mobil have been cooperating to perfect the technology.
Structural support for the sorbing compounds is provided by a highly porous silica-based ceramic. Mesoporous refers to the structure of the ceramic's internal cavities. The pores are tubular, hexagonally close-packed ( see figure below), and extremely tiny (20-200 Å in diameter), yielding a material with a surface area of approximately 1000 m²/g. That, according to C&EN, means five grams of SAMMS powder has a surface area the size of a football field.
 One example of a SAMMS nanocomposite. An hexagonally close-packed cluster of tubular pores (end view) is shown in the foreground. A single pore, in this case coated with a mercaptopropylsiloxy monolayer, is shown in the background. A model of one surfactant molcule is also shown. Photo courtesy of C&EN.
One example of a SAMMS nanocomposite. An hexagonally close-packed cluster of tubular pores (end view) is shown in the foreground. A single pore, in this case coated with a mercaptopropylsiloxy monolayer, is shown in the background. A model of one surfactant molcule is also shown. Photo courtesy of C&EN.To form the sorbent, the pores are lined with monolayers of molecules that bind to the specific pollutant in question. Such molecules have, for example, been designed to sorb mercury, lead, silver and other metals, as well as chromate, arsenate and similar anionic species.
And now cesium, which, as earlier studies had shown, can be precipitated as insoluble salts by transition metal ferrocyanide complexes. The PNNL researchers' challenge was to create a form of ferrocyanide that would be efficient, selective and practical to use. SAMMS appears to provide the solution.
As reported by C&EN and shown in the figure, below, pores in the ceramic substrate are first coated with a monolayers of molecules containing the ethylenediamine group -NHCH 2CH 2NH 2). The material is next treated with CuCl 2 , which causes the thylenediamine to wrap around copper(II) ions in groups of three so that six basic nitrogens are coordinated with the metal. The material is then treated with hexacyanoferrate(II), [Fe(CN) 6 ]4-, which binds to the copper via one of the cyano nitrogens to produce a copper ferrocyanide complex. The result: a cesium sorbent that is immobilized on the insoluble SAMMS silica structure.
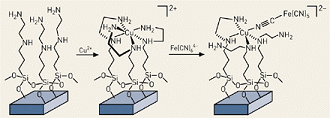 Structure of a cesium-specific sorbent on a silica substrate. Copper(II) ions form the critical link to Ce-sorbing ferrocyanides. Photo courtesy of C&EN.
Structure of a cesium-specific sorbent on a silica substrate. Copper(II) ions form the critical link to Ce-sorbing ferrocyanides. Photo courtesy of C&EN.Tests by PNNL showed that when applied to solutions that are similar in concentration to the Hanford wastes, the new SAMMS sorbent reduced cesium concentrations from 2 ppm to 4 ppb within one minute and to 1 ppb within two hours. Moreover, the sorbent was highly selective for cesium at high concentrations of sodium and potassium in both acidic and neutral solutions. Maximum loading was found to be 177 mg of cesium per gram of sorbent. Another benefit: at an estimated $50 per kilogram, the compounds will be relatively inexpensive when produced in commercial-scale quantities.
Several challenges remain. SAMMS has not yet been tested with actual nuclear waste or with 137Cs-contaminated groundwater. Also, SAMMS cannot readily tolerate highly alkaline solutions, or environments rich in fluoride. In order to treat the large quantity of waste at Hanford (and possibly groundwater, as well) it would also be beneficial to produce the materials in granular form, rather than the powders studied so far. Once these hurdles are overcome, and assuming that any of several competing sorbents don't prove to be more desirable, copper may again find itself providing a solution to one of the legacies of past nuclear technology.
References:
-
 The full article can be viewed on C&EN's Web site.
The full article can be viewed on C&EN's Web site. -
 Ironically, Dr. Dixie Lee Ray, the late Chairwoman of the Atomic Energy Commission (AEC) and one-time Governor of Washington, proposed several decades ago that 137Cs should be harvested for use from spent nuclear fuel and other radioactive waste, a view that was held in some scorn by the nuclear community at the time.
Ironically, Dr. Dixie Lee Ray, the late Chairwoman of the Atomic Energy Commission (AEC) and one-time Governor of Washington, proposed several decades ago that 137Cs should be harvested for use from spent nuclear fuel and other radioactive waste, a view that was held in some scorn by the nuclear community at the time.
Also in this Issue:
- What's a Health Nut?
- Will Copper Sop Up Radioactive Pollution
