How Hydrometallurgy and the SX/EW Process Made Copper the "Green" Metal
Copper Applications in Mining & Metallurgy
Introduction | Conventional Copper Extraction | The SX/EW Process| Bacterial Leaching | Flow Chart | ConclusionIntroduction 
Copper is traditionally known as the "red" metal after its natural color. However, it is also known as a "green" metal for the green patina that it acquires due to weathering. Indeed, patinized copper is the architectural focal point of many modern buildings for its natural look. Beyond this, however, copper can truly be cited as the "green" metal both for its role in protecting the natural environment through its use in energy-saving applications and for the achievements that have been realized in the production of the metal in an environmentally sound manner.
The energy efficiency resulting from the use of copper in high efficiency motors, electrical transformers, underground power lines, air conditioning and refrigeration equipment, electric vehicles, etc. has a significant impact on the release of green house gases resulting from the generation and use of fossil fuel based electrical power. Likewise, newly developed, high efficiency automobile radiators reduce fuel consumption by being smaller, lighter and having a lower pressure drop than their aluminum counterparts.
In both cases, the savings in energy consumed also means a conservation of our fossil fuel resources and the reduction of greenhouse gases.
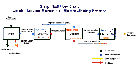
The production of copper, as in the utilization of any other natural resource, has an impact on the environment. This cannot be avoided since the earth must be disturbed in order to extract copper from it; however, the object of the copper mining industry has been to make this impact as small as possible. Significant improvements have been made in environmental impact as new technologies have been applied to the production of copper. Great strides have been accomplished in the conventional treatment of copper ores, such as at the Bingham Canyon mines, in Utah and the adjoining copper smelters. A schematic diagram of the conventional process for the production of copper and the SX/EW process can be seen here.
Conventional Copper Extraction 
Conventionally, copper is recovered by a pyrometallurgical process known as smelting. In this process copper ore is mined, crushed, ground, concentrated, smelted and refined. The mining, crushing and grinding portions of the processing are extremely energy intensive since the rock must be reduced essentially to talcum powder fineness in order to separate the copper-bearing minerals from it. To be applicable to this process, the ores must contain copper minerals in sulfide form; as mineral such as Chalcocite (Cu 2S), Chalcopyrite (CuFeS 2) and Covellite (CuS) . In the concentrating operations these minerals are separated from the gangue material of the ore, that might contain as little as 0.5% copper to form a concentrate containing 27 to 36% copper. In the smelting operation, the concentrate is fed to a smelter together with oxygen and the copper and iron sulfides are oxidized at high temperature resulting in impure molten metallic copper (97 to 99%), molten iron oxide and gaseous sulfur dioxide. The impure copper is then purified by electrolytic purification to 99.99% pure copper while the iron oxide is disposed of as slag.
Typically, in this process there is more sulfur dioxide produced by weight than there is copper. Rather than discharge the sulfur dioxide into the air, as was once the practice, the sulfur dioxide is captured and converted into sulfuric acid. In the United States, smelter-produced sulfuric acid amounts to approximately 10% of total acid production from all sources. Prior to the mid-1980s, this by-product sulfuric acid had to be sold to other industries, often at a loss due to the long shipping distances.
Beginning in the mid 1980s a new technology, commonly known as the leach-solvent extraction-electrowinning process or, SX/EW Process, was widely adopted. This new copper technology utilizes smelter acid to produce copper from oxidized ores and mine wastes. Today, worldwide, approximately 20% of all copper produced is produced by this is process. In Latin America, the total is closer to 40% whereas in the United States the total is approaching 30%.
The SX/EW Process 
The SX/EW Process is a hydrometallurgical process since it operates at ambient temperatures and the copper is in either an aqueous environment or an organic environment during its processing until it is reduced to the metal. Because of its dependence on sulfuric acid, the SX/EW Process is at present not a substitute for, but rather an adjunct to conventional smelting. However, it is also applicable in locations where smelter acid is not available by the purchase of sulfuric acid or the manufacture of sulfuric acid from sulfur or pyrite. In addition, it offers the opportunity to recover copper from an entirely different set of ores and mining byproducts than is possible by smelting; namely, oxidized materials. These may be mined copper minerals that are in oxidized form - minerals such as Azurite (2CuCO 3 · Cu(OH)3), Brochantite (CuSO 4), Chrysocolla (CuSiO 3 · 2H 2O) and Cuprite (Cu2O), residual copper in old mine waste dumps whose sulfide minerals have been oxidized by exposure to the air or sulfididic copper minerals that have been oxidized by another new technology - bacterial leaching. In addition, the process can be used to extract copper in situ. That is, without removing the material from the waste pile or from the ground. The net result of the use of this process is that copper can be produced from sources that in the past would have gone untouched, thus reducing the reliance on conventional ore bodies. Further, the process is capable of removing copper from waste materials where otherwise it would have been considered a contaminant to the environment. In the United States, for example, copper is considered to be a toxic material released to the environment once it is mined under Emergency Planning and Community Right-to-Know Act (EPCRA) and the Environmental Protection Agency's Toxic Release Inventory (TRI). Copper mine dumps and flotation tailings constitute a significant inventory of copper that is considered to be a contaminant to the environment under TRI.
The SX/EW process, itself, has very little environmental impact because its liquid streams are very easily contained, There is no effluent inasmuch as all impurities are returned to the site where they originated and the sulfuric acid is eventually neutralized by the limestone in the ore body or waste dump where it is deposited as calcium sulfate (gypsum) - a very insoluble substance.
The SX/EW Process has its roots in analytical chemistry where it is used to separate one metallic ion from another. It was first used as a large-scale process during World War II for the recovery of uranium from its ores. The key to the process is the development of organic extractants that are specific to the metal to be extracted. The first extractant that was specific for copper and used on a commercial scale was developed by General Mills Corporation and sold under the name LIX 64® (LIX for Liquid Ion Exchange and Roman for 1959 - the year of the first patent.) Ranchers Exploration and Development Corporation at its Bluebird Mine in Arizona in 1968 first demonstrated it on a large scale.
The process involves leaching the material with a weak acid solution. This solution, known as pregnant liquor, is recovered and then contacted with an organic solvent, referred to as the extractant, in the solvent extraction stage (SX). Here the copper is extracted away from the aqueous phase leaving behind most of the impurities that were in the leach solution. Since the copper ion is exchanged for hydrogen ion, the aqueous phase is returned to its original acidity and recycled to the leaching step of the process. Meanwhile, the copper-bearing organic phase is stripped of its copper by contacting it with a strongly acidified aqueous solution at which time the copper is moved to the aqueous phase while the organic phase is reconstituted in its hydrogen form. The copper-bearing aqueous phase is advanced to the electrowinning (EW) stage of the process while the barren organic phase is returned to the extraction stage of the process. In the electrowinning stage of the process the copper is reduced electrochemically from copper sulfate in solution to a metallic copper cathode. Electrowon copper cathodes are as pure as or purer than electrorefined cathodes from the smelting process. Thus they are well received by the market.
Whereas the conventional process requires an estimated 65 MJ/kg of energy (not including scrap recycling ) the SX/EW process requires an estimated 15 MJ/kg (from heap or dump leaching) to 36 MJ/kg. (from mined and crushed ore). In addition to variations in energy consumption based on the origin of the material leached, there are also variations based on the particular circumstances of the installation. In the United States, for example, the energy requirement for SX/EW processing of heap or dump leaching ranges from an estimated 10 MJ/kg to 25 MJ/kg - the difference being largely the pumping costs. In the case of the higher energy consumer, the pregnant liquor is pumped five miles from the leach site to the SX/EW plant.
The electrowinning of copper requires considerably more electrical energy than does the electrorefining process - an average of about 8 MJ/kg for electrowinning vs about 1.5 MJ/kg for electrorefining. In electrowinning the copper must be reduced from the cupric form to metal; whereas, in electrorefining the copper is already in metallic form and is merely transported from the anode to the cathode to purify it. Steps are under development to reduce the energy requirement of electrowinning by about 40% by a modification of the anode reaction.
Another advantage of the process is its low capital investment requirement relative to the smelting process and its ability to be operated economically in a small scale. In China, for example, where copper deposits are not plentiful and tend to be small, there are 40 to 50 "mom & pop" leaching operations involving SX/EW.
Bacterial Leaching 
A second technology that has aided in the production of environmentally clean copper is that of bacterial leaching or bioleaching. This is used as an adjunct to the SX/EW process in situations where sulfide copper minerals must be leached. Modern commercial application of bacterial leaching began in the 1950s at Kennecott's Bingham mine near Salt Lake City, Utah. It was noticed that blue copper-containing solutions were running out of waste piles that contained copper sulfide minerals - a condition that should not have happened in the absence of powerful oxidizing agents and acid. On investigation it was found that naturally occurring bacteria were oxidizing iron sulfides and the resulting ferric sulfate was acting as an oxidizer and leachant for copper sulfides. These bacteria were given the name - ferrooxidans for their action in oxidizing iron sulfides. A second set of bacteria were also identified and given the name - thiooxidans for their action in oxidizing sulfur to yield sulfuric acid.
Bacterial leaching, combined with SX/EW, offers a method of exploiting small ore bodies with a minimum of capital investment. Most commercial operations leaching copper from ore dumps are located in the Southern Hemisphere in Australia, Chile, Myanmar and Peru. The process consists of injecting the material to be leached with cultivated strains of appropriate bacteria and maintaining conditions that are conducive to their effective operation and propagation. Air, for instance, is blown into the heap through air lines situated under the leach pad. Since these naturally-occurring bacteria are present nearly everywhere, they undoubtedly play a role in all acid leaching operations; however, these are not considered bacterial leaching since neither cultivated bacteria nor air are added and acid is applied. While copper bacterial leaching thus far has been confined to the leaching of ore, pilot plant tests are underway for the leaching of chalcopyrite concentrates that would normally be processed by smelting.
SX/EW Flow Chart 
Conclusion 
The SX/EW process has provided the copper industry with a tool that makes the extraction of copper from its ores significantly more environmentally friendly than by the use of the conventional smelting process. While the SX/EW process was adopted by the industry to take benefit from the sulfuric acid that the smelting process produced steps are being take to divorce the two. The Phelps Dodge Mining Company, for example, at its Morenci, Arizona mine, has completely disassembled its smelter and has converted the mine to a mine-for-leach operation. Details of this are given in a separate Innovations paper.
Contacts and References 
R. Brantley Sudderth, Vice President - Cognis Corporation, Tucson, Arizona - SX reagents
Sharon K. Young, President, VersiTech Corporation, Tucson, Arizona - SX/EW processing
CRU, The Ten Year Outlook for Copper, 1998, CRU International Ltd., London, UK. and Exton, PA, USA. Confidential Report
BHA, The Impact of SxEw Production on the Copper Industry, 1994, Brook Hunt & Associates, Ltd., Addlestone, Surry, UK. Confidential Report
Brierley, J.A., Expanding role of microbiology in metallurgical processes, Mining Engineering 52, No. 11, 2000, pp 49 - 53.
Tilton, J. E. and H. H. Lansberg, Innovation, Productivity Growth and the Survival of the U.S. Copper Industry,1997, Discussion Paper No. 97-41, Resources for the Future, Washington, D.C.
Also in this Issue:
- Introduction to Copper: Applications
- Introduction to Copper: Types of Copper
- Introduction to Copper: Mining & Extraction
- Introduction to Copper: Fact Sheets
- Phelps Dodge Morenci Has Converted All Copper Production to Mine-for-Leach
- How Hydrometallurgy and the SX/EW Process Made Copper the "Green" Metal
- Introduction to Copper: Hot Links & Further Reading